Di Cai*, Shouye Yang
State Key Laboratory of Marine Geology, Tongji University, 1239 Siping Road, Shanghai 200092, PR China
Abstract
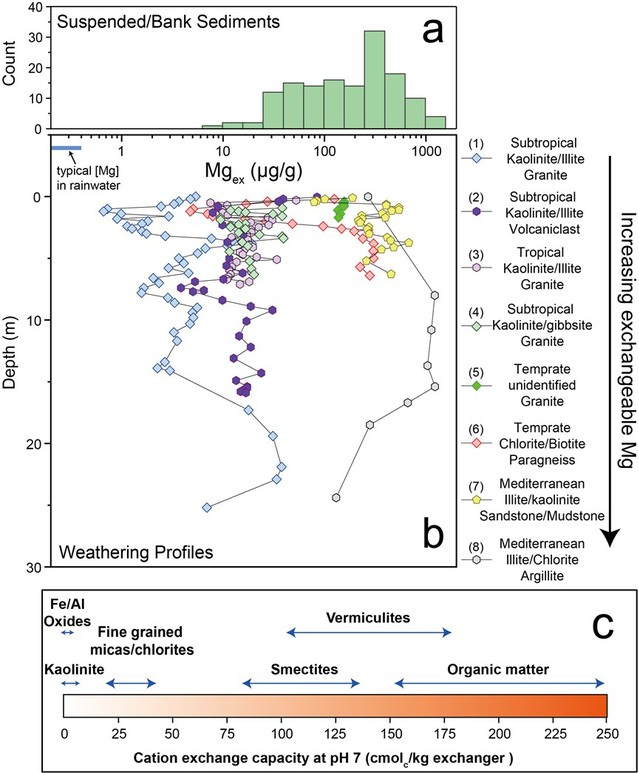
Investigations into the interaction between rock (sediment) and water at Earth’s surface have primarily focused on mineral dissolution/precipitation processes. While the presence of exchangeable cations on mineral surfaces has long been recognized, the coupled chemical evolution of this labile pool with surface water remains less understood. In this study, we demonstrate that Mg isotopes (expressed as δ value-δ26Mg) serve as an effective proxy for tracing cation exchange processes, as our batch exchange experiments and field investigations showed marginal Mg isotope fractionation (<0.2 ‰) between concomitant dissolved Mg and exchangeable Mg. This finding suggests that Mg isotope exchange follows a simple mixing process, resulting in nearly identical δ26Mg values between dissolved and exchangeable phases once equilibrium is achieved. Thus, the potential for exchangeable Mg to alter the δ²⁶Mg of water—or vice versa—depends on the relative masses of Mg in these two phases. We further investigated the relative proportions of dissolved and exchangeable Mg across the surface sediment cycling pathway, from regolith to rivers and ultimately to estuaries. Our compiled data suggest that the exchangeable Mg pools in regolith from various geological settings are substantial enough to buffer the concentration and isotopic composition of Mg in infiltrating water, reducing their variations in runoff under changing hydrological conditions. In river channels, however, exchangeable Mg in suspended sediments accounts for a small fraction (~6 ± 1.5%) of total dissolved Mg globally, meaning that exchange reactions are expected to have little impact on water chemistry as SPM enter the river channels or the ocean.